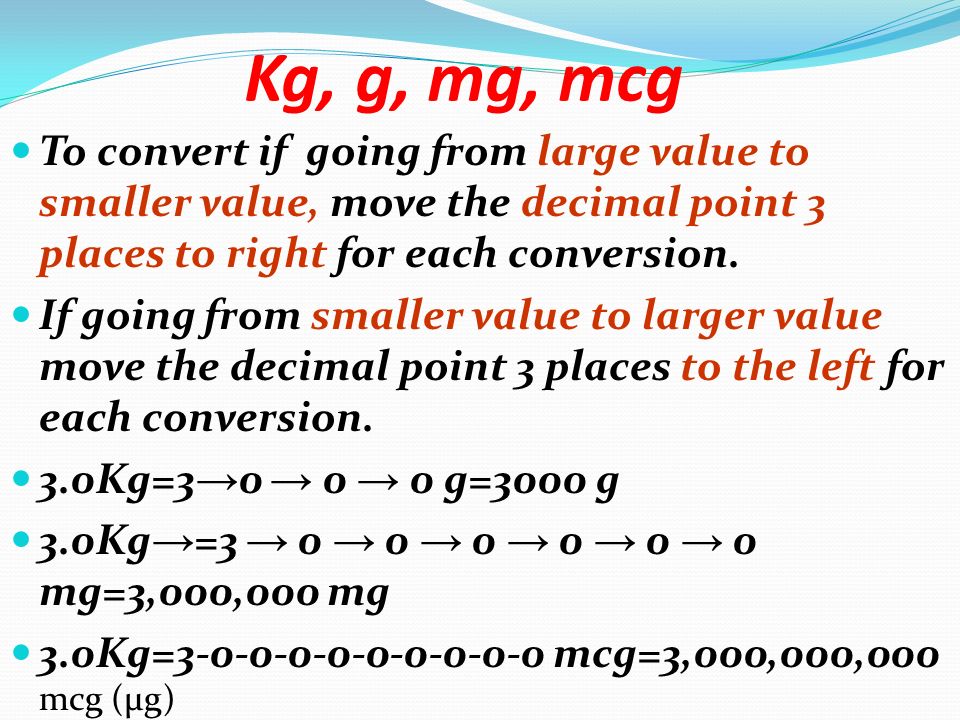
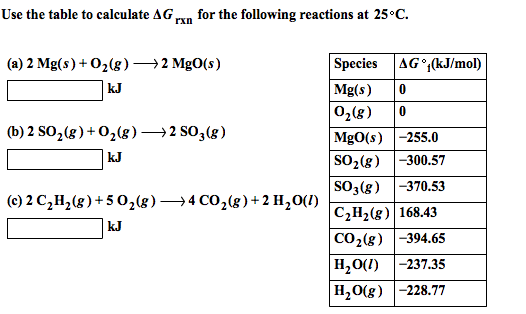
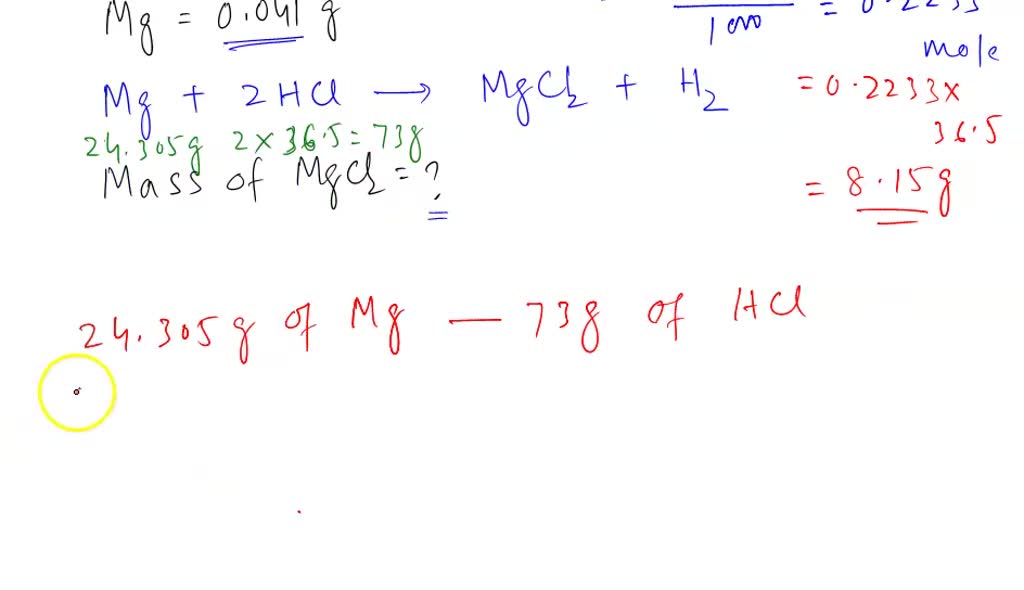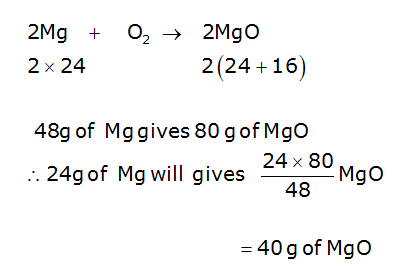Printable Grams to Milligrams Conversion Chart | Baking conversion chart, Baking conversions, Cooking conversions
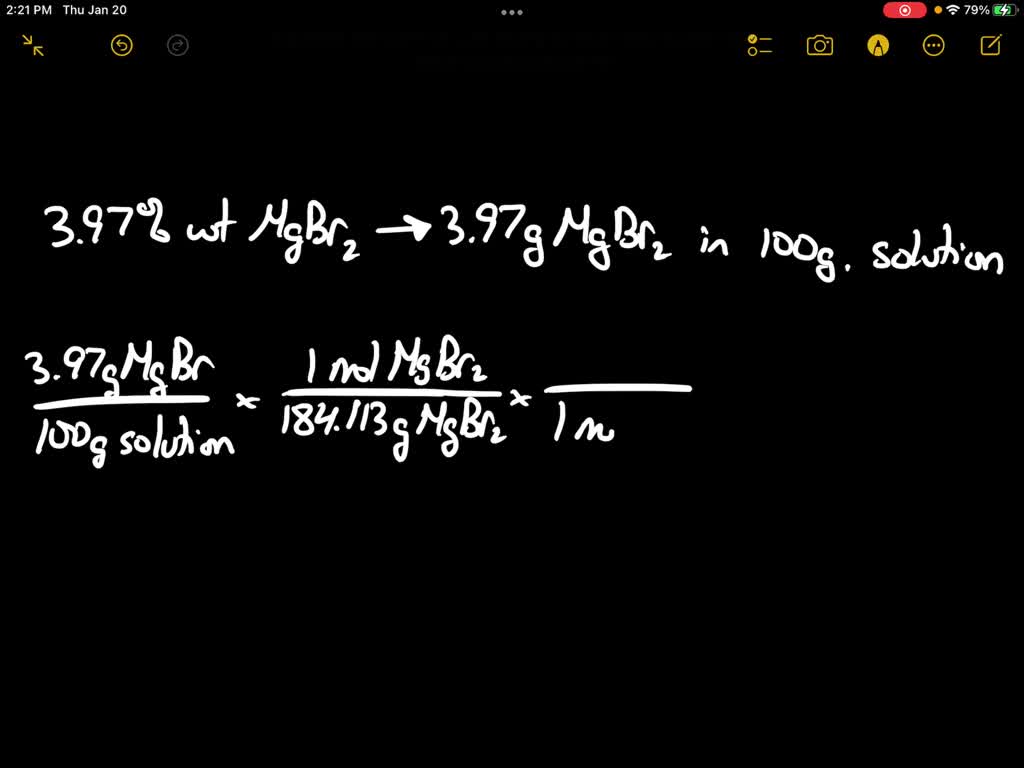
SOLVED: Calculate the concentration of Br− ( MW=79.904 g/mol ), in milligrams per milliliter (mg/mL), in an aqueous solution that is 3.97 wt% MgBr2 (MW=184.113 g/mol) . The density of this solution

1 g of Mg is burnt in a closed vessel containing 0.5 g of O2 . Which reactant is limiting how much of the reagent and excess reactant will be left?

Amazon.com: Reflex 500g / 0.01g Digital Pocket Wireless Smart Food Kitchen Scale Grams and Ounces USB Rechargeable, Portable, Accurate, Metal Stainless Steel Surface, Keto Calculator, Baking, Counter, Reloading: Digital Kitchen Scales: Home
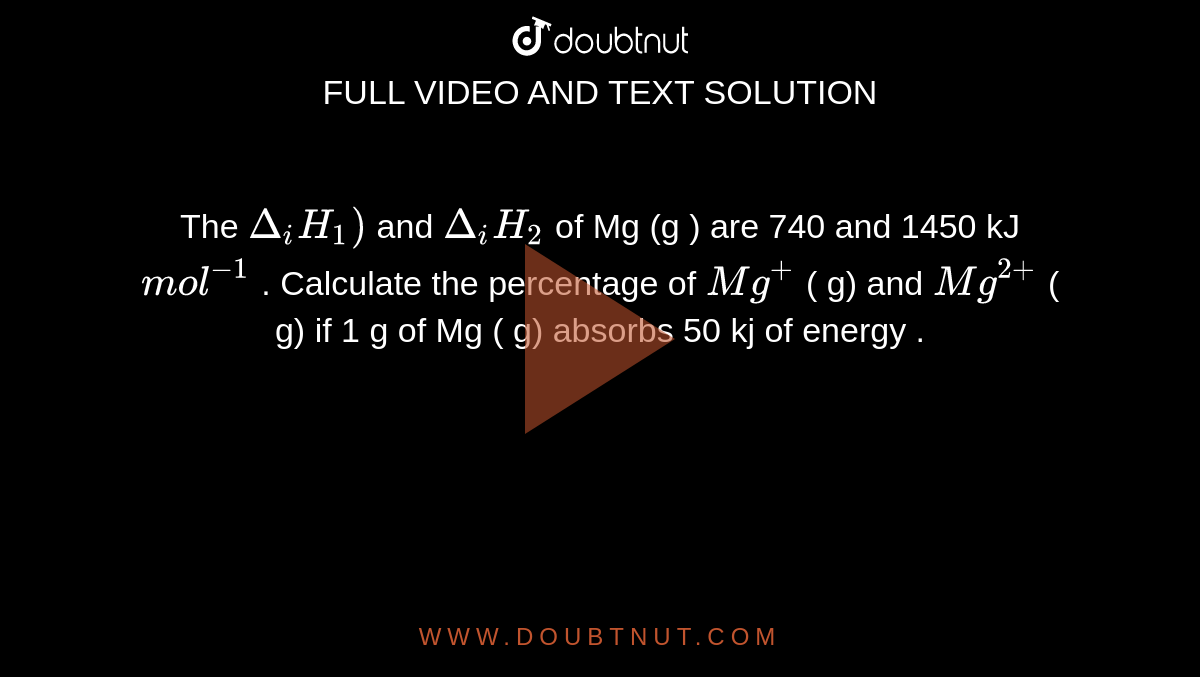
The IE1 " and " IE2 of Mg (g) are 740 and 1450 kJ "mol:^(-1) . Calculate the percentage of Mg^(+) (g) and Mg^(2+) (g) if 1g of Mg (g) absorbs 50 kJ of energy.

a) Calculate ΔrG^0 for the reaction Mg (s) + Cu^2 + (aq) →Mg^2 + (aq) + Cu (s) .Given: Ecell^0 = 2.71V,1F = 96500Cmol^-1 (b) Name the type of cell that was