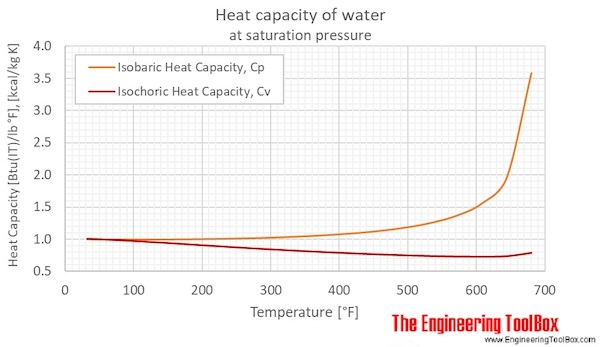
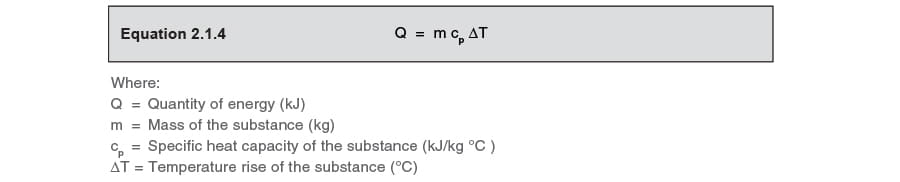
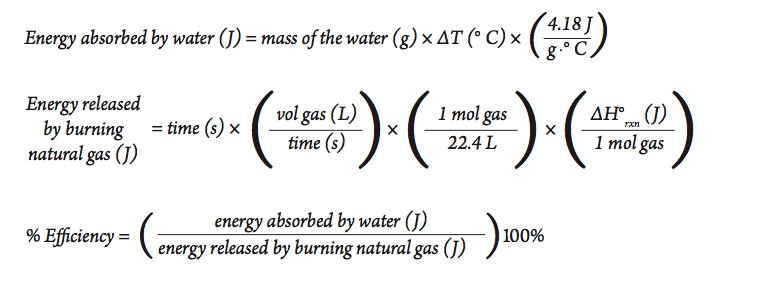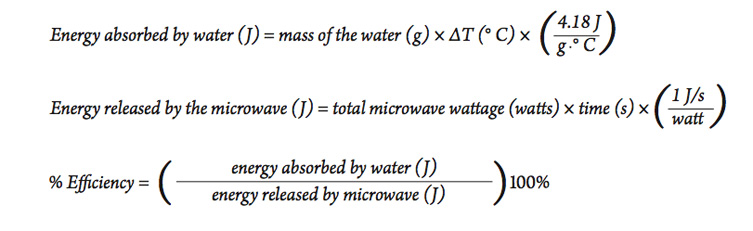SOLVED: Calculate the energy needed to heat 250.0 g of water from 25 degrees * C to 175 degrees * C . Specific heats : water=4.18 J/g^ C,steam=1.99 J/g^ C . A )

Question Video: Finding the Amount of Energy Needed to Change the State of Water from Liquid to Gas | Nagwa

Calculate the heat energy required to raise the temperature of 2 kg of water from 10^@C to 50^@C. Specific heat capacity of water is 4200 JKg^(-1) K^(-1)
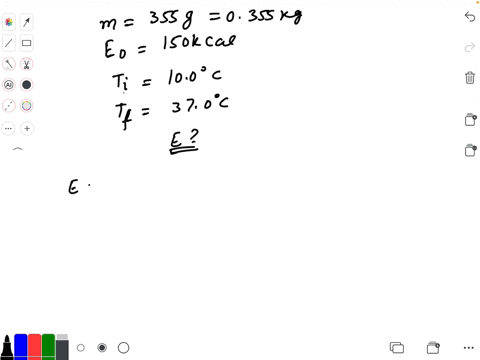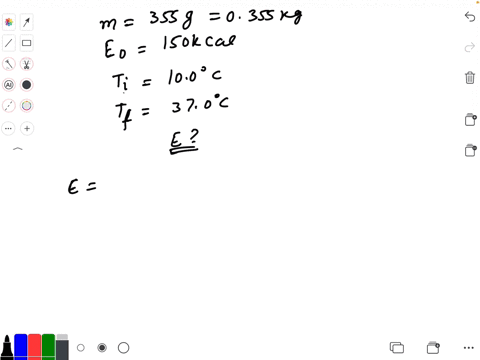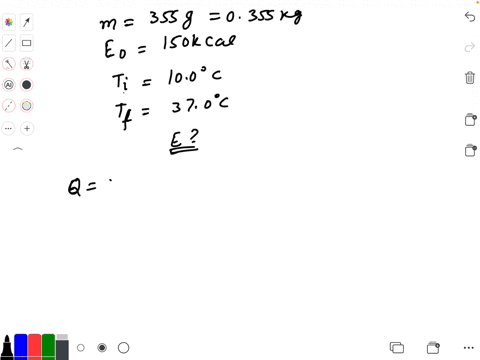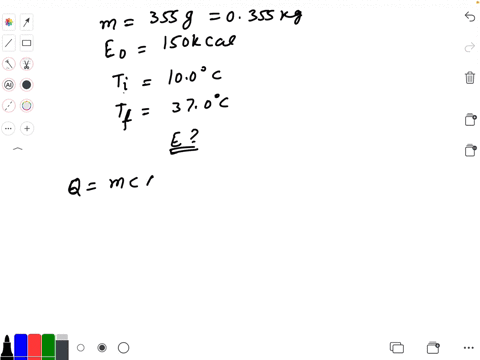
SOLVED: Calculate The Energy Needed To Heat The Cube Of Silver; With A Volume Of Cm? From 14 C To 26 Refer To The Tables Express The Heat In Calories | C&c

SOLVED: We'll begin by calculating the energy absorbed by the water. The water increased in temperature from 21.4 °C to 39.2 °C. What quantity of heat did it absorb? Recall from the

Heat and Temperature Heat is a form of energy, and is measured in Joules (J). Temperature is different from heat. Temperature is a measure of how hot or. - ppt video online

The heat energy required to raise the temperature of 2kg of water from 10^0C to 50^0C is (Specific heat capacity of water is 4200 J Kg^0C^-1)
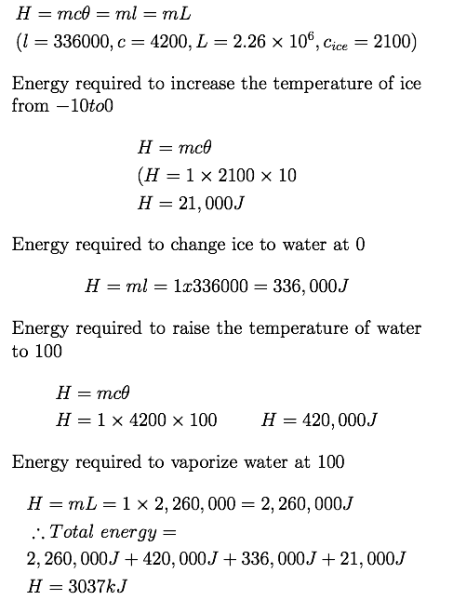
calculate the total energy required to evaporate completely 1kg of ice that is initially at... - Myschool

Calorie (energy) Calculations A calorie is defined as the amount of energy it takes to raise the temperature of one gram of water by one degree Celsius. - ppt download